Space Ranger1.3, printed on 03/26/2025
| Space Ranger v1.3 or later is required to analyze FFPE data with spaceranger count and to detect Dual Index Kit TS Set A when demultiplexing with spaceranger mkfastq. |
Space Ranger's pipelines analyze sequencing data produced from Visium Spatial Gene Expression for formalin fixed paraffin embedded (FFPE) and Fresh Frozen (FF) tissue samples. The workflow diagram shows all the required inputs and the corresponding spaceranger flags for FFPE tissue samples:
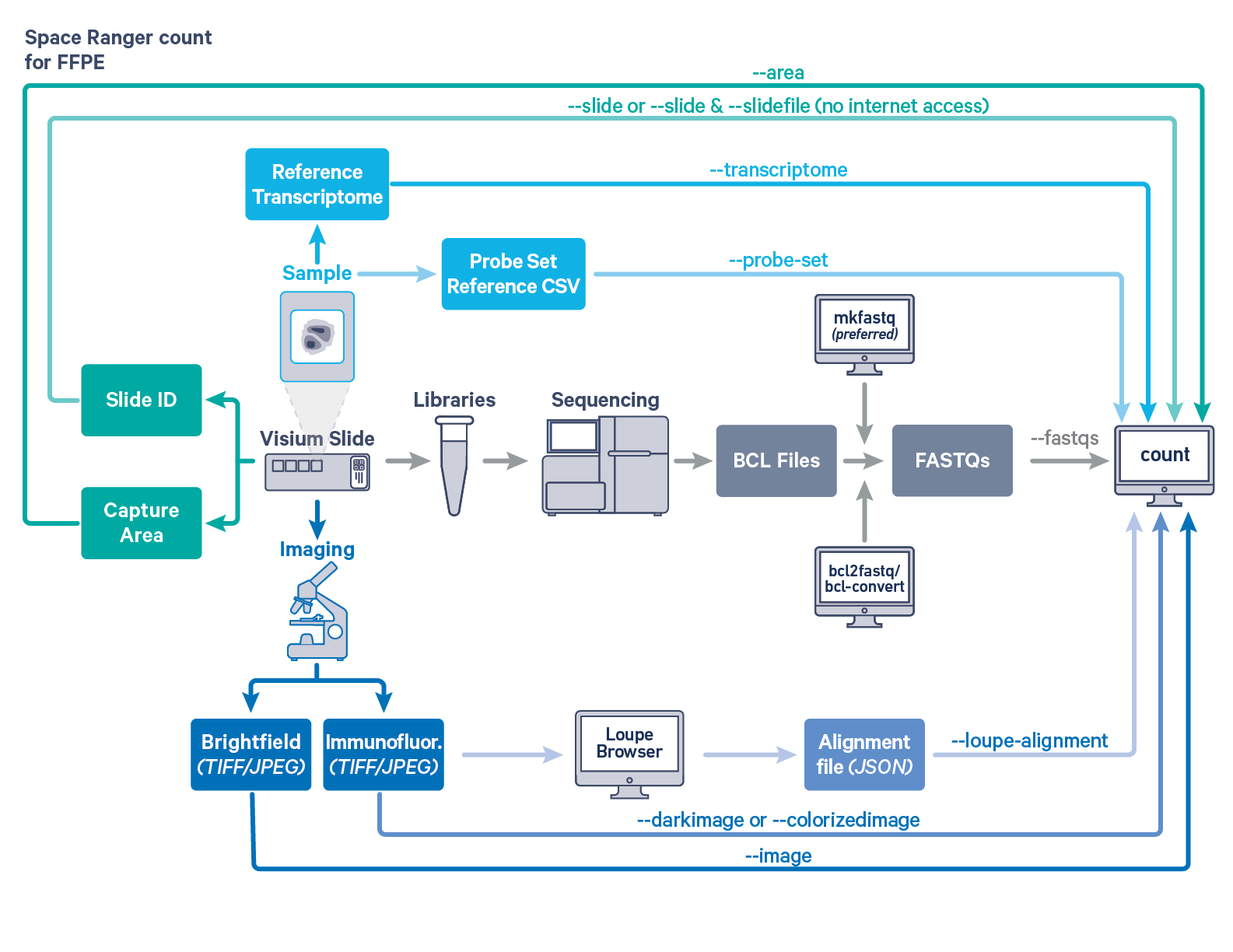
The spaceranger count is run on each individual Capture Area in the Visium slide. The required inputs are
--fastqs)
TIFF or JPEG format
--image for brightfield image--darkimage for dark background fluorescence image--colorizedimage for composite colored fluorescence image--slide & --area if spaceranger has access to internet--slidefile, --slide & --area if spaceranger has no access to internet. The slide layout file is directly downloaded--unknown-slide if Visium slide details are unknown--transcriptome)--probe-set flagFor a list of accepted arguments, see the Command Line Argument Reference below, or run spaceranger count --help.
Select the tab corresponding to the imaging workflow.
$ cd /home/jdoe/runs $ spaceranger count --id=sample345 \ #Output directory --transcriptome=/opt/refdata/GRCh38-2020-A \ #Path to Reference --probe-set=/opt/spaceranger-1.3.0/probe_set/Visium_Human_Transcriptome_Probe_Set_v1.0_GRCh38-2020-A.csv\ #Path to Probe Set --fastqs=/home/jdoe/runs/HAWT7ADXX/outs/fastq_path \ #Path to FASTQs --sample=mysample \ #Sample name from FASTQ filename --image=/home/jdoe/runs/images/sample345TIFF \ #Path to brightfield image input --slide=V19J01-123 \ #Slide ID --area=A1 \ #Capture area --localcores=8 \ #Allowed cores in localmode --localmem=64 #Allowed memory (GB) in localmode
JSON file generated in Loupe Browser on a brightfield image input, run spaceranger count with the following arguments. The code in red indicates user specific inputs.
$ cd /home/jdoe/runs $ spaceranger count --id=sample345 \ #Output directory --transcriptome=/opt/refdata/GRCh38-2020-A \ #Path to Reference --probe-set=/opt/spaceranger-1.3.0/probe_set/Visium_Human_Transcriptome_Probe_Set_v1.0_GRCh38-2020-A.csv\ #Path to Probe Set --fastqs=/home/jdoe/runs/HAWT7ADXX/outs/fastq_path \ #Path to FASTQs --sample=mysample \ #Sample name from FASTQ filename --image=/home/jdoe/runs/images/sample345TIFF \ #Path to brightfield image input --slide=V19J01-123 \ #Slide ID --area=A1 \ #Capture area --loupe-alignment=sample345.json \ #Manual alignment file --localcores=8 \ #Allowed cores in localmode --localmem=64 #Allowed memory (GB) in localmodeThe arguments to specify the fluorescence image can be either
--darkimage or --colorizedimage depending on the image format. Details about the different supported fluorescence image formats are described in Image Recommendations.
Martian Runtime - 4.0.5 Running preflight checks (please wait)... Checking sample info... Checking FASTQ folder... Checking reference... Checking reference_path Checking optional arguments... ...
Probe set reference CSV file for human can be found in the probe_sets directory in the Space Ranger package.
spaceranger-1.3.0/probe_sets/ └── Visium_Human_Transcriptome_Probe_Set_v1.0_GRCh38-2020-A.csv
The CSV files for both human and mouse
A successful spaceranger count run concludes with a message similar to this:
Outputs: - Run summary HTML: /opt/sample345/outs/web_summary.html - Outputs of spatial pipeline: /opt/sample345/outs/spatial - Run summary CSV: /opt/sample345/outs/metrics_summary.csv - BAM: /opt/sample345/outs/possorted_genome_bam.bam - BAM index: /opt/sample345/outs/possorted_genome_bam.bam.bai - Filtered feature-barcode matrices MEX: /opt/sample345/outs/filtered_feature_bc_matrix - Filtered feature-barcode matrices HDF5: /opt/sample345/outs/filtered_feature_bc_matrix.h5 - Unfiltered feature-barcode matrices MEX: /opt/sample345/outs/raw_feature_bc_matrix - Unfiltered feature-barcode matrices HDF5: /opt/sample345/outs/raw_feature_bc_matrix.h5 - Secondary analysis output CSV: /opt/sample345/outs/analysis - Per-molecule read information: /opt/sample345/outs/molecule_info.h5 - Loupe Browser file: /opt/sample345/outs/cloupe.cloupe - Spatial Enrichment using Moran's I file: /opt/sample345/outs/spatial_enrichment.csv - Probe Set Reference CSV file /opt/sample345/outs/probe_set.csv Pipestance completed successfully!
The output of the pipeline is contained in a folder named with the sample ID you specified (such as sample345). If this folder already exists, in a rerun with the original parameters, spaceranger will assume it is an existing pipestance and attempt to resume running it. The subfolder named outs contains the main pipeline output files.
outs ├── analysis │ ├── clustering │ ├── diffexp │ ├── pca │ ├── tsne │ └── umap ├── cloupe.cloupe ├── filtered_feature_bc_matrix │ ├── barcodes.tsv.gz │ ├── features.tsv.gz │ └── matrix.mtx.gz ├── filtered_feature_bc_matrix.h5 ├── metrics_summary.csv ├── molecule_info.h5 ├── possorted_genome_bam.bam ├── possorted_genome_bam.bam.bai ├── probe_set.csv ├── raw_feature_bc_matrix │ ├── barcodes.tsv.gz │ ├── features.tsv.gz │ └── matrix.mtx.gz ├── raw_feature_bc_matrix.h5 ├── spatial │ ├── aligned_fiducials.jpg │ ├── detected_tissue_image.jpg │ ├── scalefactors_json.json │ ├── tissue_hires_image.png │ ├── tissue_lowres_image.png │ └── tissue_positions_list.csv ├── spatial_enrichment.csv └── web_summary.html
| File Name | Description |
|---|---|
web_summary.html | Run summary metrics and charts in HTML format |
cloupe.cloupe | Loupe Browser visualization and analysis file |
spatial/aligned_fiducials.jpg | Aligned fiducials QC image |
spatial/detected_tissue_image.jpg | Detected tissue QC image |
spatial/tissue_hires_image.png | Full resolution image downsampled to 2k pixels on the longest dimension |
spatial/tissue_lowres_image.png | Full resolution image downsampled to 600 pixels on the longest dimension |
spatial/tissue_positions_list.csv | CSV containing spot barcode, if the spot was called under (1) or out (0) of tissue, the array position, image pixel position x, and image pixel position y for the full resolution image |
spatial/scalefactors_json.json | Contains spot diameter estimation in pixels for the full resolution original image, tissue_hires_scalef which is the spot position multiplier in pixels for the high resolution image, fiducial spot diameter estimation in pixels for the full resolution original image, and tissue_hires_scalef which is the spot position multiplier in pixels for the low resolution image |
spatial_enrichment.csv | Feature spatial autocorrelation analysis using Moran's I in csv format |
analysis | Secondary analysis data including dimensionality reduction, spot clustering, and differential expression |
metrics_summary.csv | Run summary metrics in CSV format |
probe_set.csv | Probe set reference CSV file. Present only for Visium FFPE data |
possorted_genome_bam.bam | Reads aligned to the genome and transcriptome annotated with barcode information |
possorted_genome_bam.bam.bai | Index for possorted_genome_bam.bam |
filtered_feature_bc_matrix | Filtered feature-barcode matrices containing only spot barcodes in MEX format |
filtered_feature_bc_matrix.h5 | Filtered feature-barcode matrices containing only spot barcodes in HDF5 format |
raw_feature_bc_matrices | Unfiltered feature-barcode matrices containing all barcodes in MEX format |
raw_feature_bc_matrix.h5 | Unfiltered feature-barcode matrices containing all barcodes in HDF5 format |
molecule_info.h5 | Molecule-level information used by secondary analysis spaceranger pipelines including aggr, targeted-compare and targeted-depth |
Once spaceranger count has successfully completed, you can further explore the results by
.cloupe file in
Loupe BrowserThe flags unique to FFPE analysis are highlighted in bold.
| Argument | Description |
|---|---|
--id | Required. A unique run ID string (such as sample345). |
--fastqs | Required. Either: Path of the fastq_path folder generated by spaceranger mkfastq (such as /home/jdoe/runs/HAWT7ADXX/outs/fastq_path). This contains a directory hierarchy that spaceranger count will automatically traverse.- OR - Any folder containing fastq files, for example if the fastq files were generated by a service provider and delivered outside the context of the mkfastq output directory structure. Can take multiple comma-separated paths, which is helpful if the same library was sequenced on multiple flowcells. Doing this will treat all reads from the library, across flowcells, as one sample. |
--sample | Optional. Sample name as specified in the sample sheet supplied to spaceranger mkfastq.
Can take multiple comma-separated values, which is helpful if the same library was sequenced on multiple flowcells and the sample name used (and therefore fastq file prefix) is not identical between them. Doing this will treat all reads from the library, across flowcells, as one sample. Allowable characters in sample names are letters, numbers, hyphens, and underscores. |
--probe-set | Required to enable FFPE analysis. Path to the probe set reference CSV file (such as /opt/spaceranger-1.3.1/probe_sets/Visium_Human_Transcriptome_Probe_Set_v1.0_GRCh38-2020-A.csv). |
--transcriptome | Required. Path to the Space Ranger compatible transcriptome reference (such as /opt/GRCh38-2020-A). |
--image | Required for brightfield image input. Brightfield tissue H&E image in JPEG or TIFF format. For more information on image file constraints, encoding and formats refer the Image Recommendations. |
--darkimage | Required for dark background fluorescence image input. Multi-channel, dark-background fluorescence image as either a single, multi-layer TIFF file or as multiple TIFF or JPEG files (which can provided as a comma separated list or by invoking the --darkimage parameter multiple times). Details on image file constraints, encoding and formats are described in the Image Recommendations. |
--colorizedimage | Required for color composite fluorescence image input. A color composite of one or more fluorescence image channels saved as a single-page, single-file color TIFF or JPEG. Please see the Image Recommendations for information on input image file formats. |
--slide | Visium slide serial number. Required unless --unknown-slide is passed. |
--area | Visium capture area identifier. Required unless --unknown-slide is passed. Options for Visium are A1, B1, C1, D1. |
--slidefile | Slide layout file indicating capture spot and fiducial spot positions. Required when no internet access unless --unknown-slide is passed. |
--reorient-images | Recommended. Use with automatic image alignment to specify that images may not be in canonical orientation with the hourglass in the top left corner of the image. The automatic fiducial alignment will attempt to align any rotation or mirroring of the image. |
--loupe-alignment | Required for manual alignment. Alignment file produced by the manual Loupe alignment step. A --image, --darkimage or --colorizedimage, the same image(s) that were used for manual alignment, must be supplied in this case. |
--unknown-slide | Optional. Set this if the slide serial number and area identifier are unknown. Setting this will cause Space Ranger to use default spot positions. Not compatible with --slide, --area, or --slidefile. |
--no-probe-filter | Optional for FFPE analysis. Include all non-deprecated probes listed in the probe set reference CSV file. Probes that are predicted to have off-target activity to homologous genes are excluded from analysis by default. Setting --no-probe-filter will result in UMI counts from all non-deprecated probes, including those with predicted off-target activity, to be used in the analysis. An alternative and preferable method to including all probes is described in this article. Probes whose ID is prefixed with DEPRECATED are always excluded from the analysis. |
--no-bam | Optional. Do not generate a BAM file. |
--nosecondary | Optional. Disable secondary analysis, such as dimensionality reduction, clustering, spatial enrichment calculation (Moran's I) and visualization. |
--r1-length | Optional. Limit the length of the input R1 sequence to the first N bases where N is the value supplied by the user. Note that the length includes the Barcode and UMI sequences so do not set this below 28. This and --r2-length are useful for determining the optimal read length for sequencing. |
--r2-length | Optional. Limit the length of the input R2 sequence to the first N bases where N is the value supplied by the user. |
--lanes | Optional. Lanes associated with this sample. |
--localcores | Recommended when run in localmode. Restricts spaceranger to use specified number of cores to execute pipeline stages. By default, spaceranger will use all of the cores available on your system. |
--localmem | Recommended when run in localmode. Restricts spaceranger to use specified amount of memory (in GB) to execute pipeline stages. By default, spaceranger will use 90% of the memory available on your system. |